In the past month, 341 interventional studies investigating hundreds of new candidate treatments and combination therapies were added to the ClinicalTrials.gov registry. Convalescent plasma has increasing in popularity, while lopinavir+ritonavir and remdesivir less so. The number of trials on favipiravir have increased dramatically since our last report. Hydroxychloroquine or chloroquine alone or plus azithromycin, mesenchymal stem cells, and tocilizumab remain popular in registered interventional studies.
Recently, some randomized clinical trials (RCTs) have released their results, and a few major health agencies have updated their guidelines based on the increasing evidence. However, more high-quality evidence from RCTs are still urgently needed.
We have updated a previously published OE Original, Emerging Treatments in Fight Against COVID-19, to offer the latest information and evidence on potential treatments against COVID-19.
What’s New: Ongoing Trials
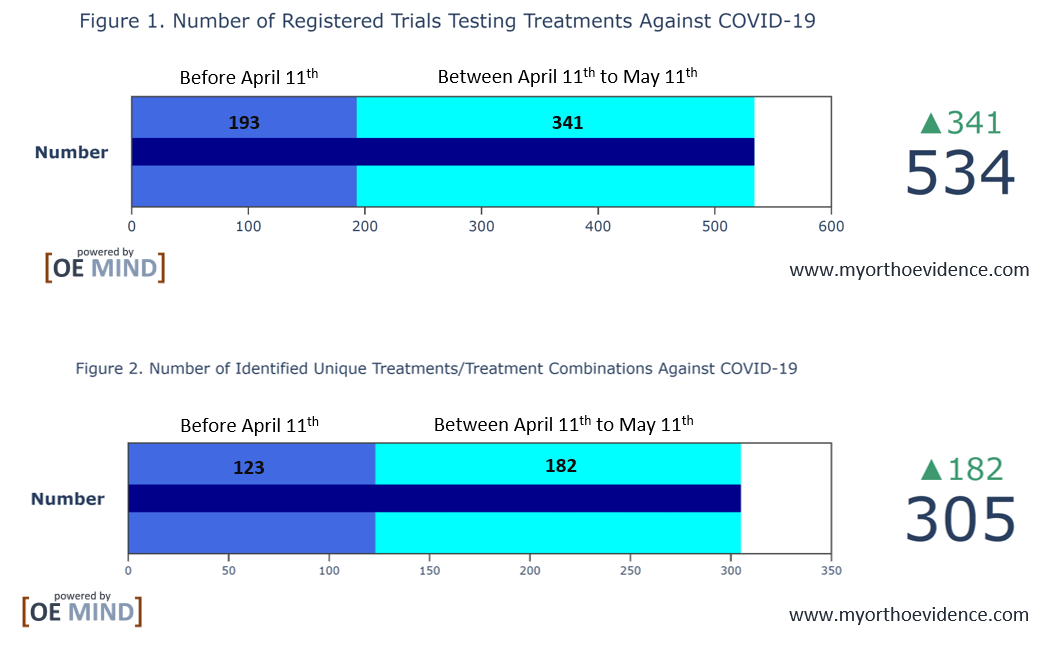
Between April 11th and May 11th, 2020, over 300 new interventional studies aimed at treatments for COVID-19 and associated conditions have been registered on ClinicalTrials.gov, from which almost 200 new treatments or treatment combinations were identified (Figure 1 and 2).
The Wheel of (Spending) Fortunes for COVID-19 Treatments
Using OrthoEvidence (OE MIND) tools, we identified 305 treatments currently under investigation (Figure 3). The size of the areas in the chart corresponds to the number of trials in which the treatment is being investigated.

Top Trending Treatments
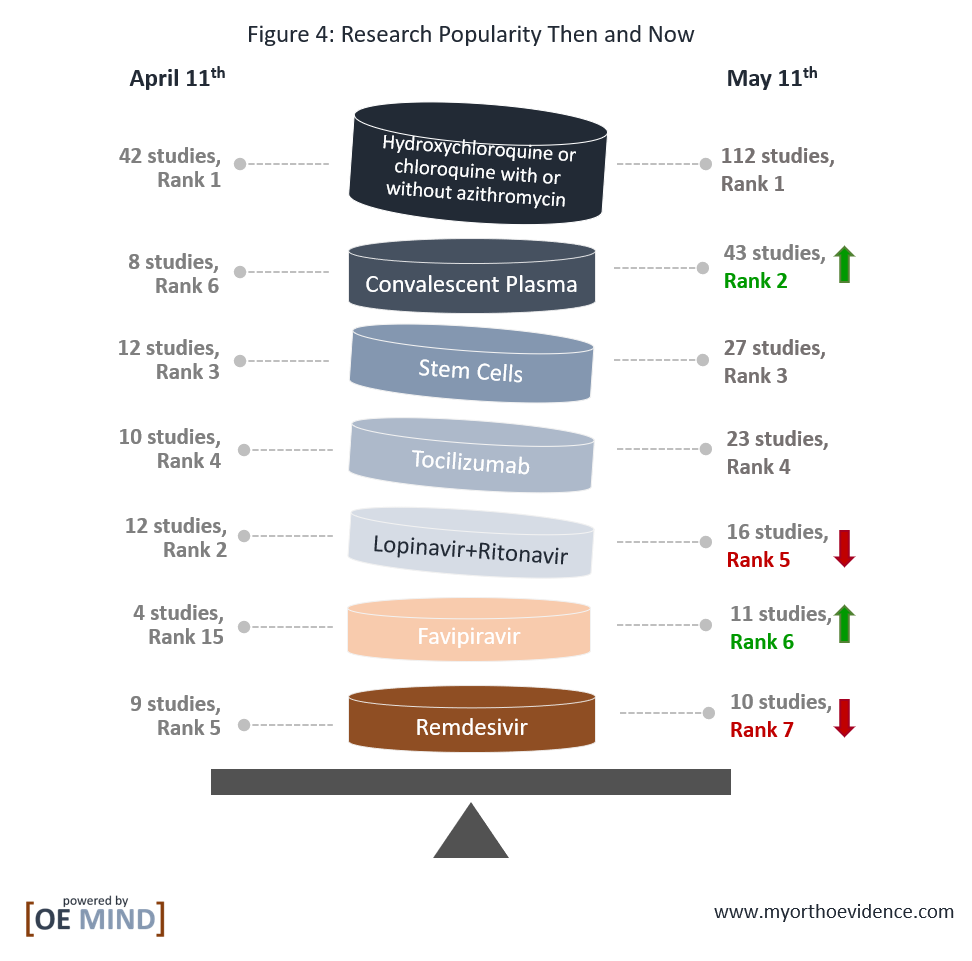
Compared to one month ago, hydroxychloroquine or chloroquine with or without azithromycin, stem cells, and tocilizumab are still the most popular. Convalescent plasma has now become the second most popular treatment among interventional trials, whereas lopinavir+ritonavir and remdesivir have become less so. Notably, favipiravir has gained much popularity in the past month (Figure 4).
A Sobering Reality: Evidence-Based Guidelines
The major agencies who recently updated their guidelines include: the Infectious Diseases Society of America (IDSA) (Bhimraj et al., 2020 and updated on April 21st, 2020); the National Institute of Health (NIH) (National Institute of Health, 2020 and updated on May 12th, 2020); the Canadian Medical Association (CMA) (Ye et al., 2020 and released on April 29th, 2020), and the Australia and New Zealand Intensive Care Society (ANZICS) (Australia and New Zealand Intensive Care Society, 2020 and updated on April 15th,2020).
Hydroxychloroquine/Chloroquine with or without azithromycin (112 studies, Table 1)
| Table 1. An update on hydroxychloroquine/chloroquine with or without azithromycin | |
|
RCTs with results |
1. A pilot RCT found no significant difference in negative conversion rate of SARS-CoV-2 nucleic acid between patients treated with hydroxychloroquine vs. standard care (Chen, J. et al., 2020).
2. A non-peer-reviewed RCT published on MedRxiv showed that hydroxychloroquine could significantly shorten the time to clinical recovery and advance the resolution of pneumonia (Chen, Z. et al., 2020). 3. Another RCT published on MedRxiv showed that hydroxychloroquine didn’t significantly clear the SARS-CoV-2 or improve outcomes for patients with mild to moderate COVID-19, compared to standard care alone (Tang, W. et al., 2020). |
|
Recent Guidelines |
1. IDSA: Among hospitalized patients with COVID-19, hydroxychloroquine/chloroquine alone or plus azithromycin are only recommended in the context of clinical trials.
2. NIH: Insufficient evidence to recommend for/against the use of hydroxychloroquine/chloroquine. 3. CMA recommends against the use of hydroxychloroquine in patients with COVID-19 (weak recommendation). 4. ANZICS: Not provided. |
Convalescent Plasma (43 studies, Table 2)
| Table 2. An update on convalescent plasma | |
|
RCTs with results |
1. No RCT results available.
2. A recent systematic review done by Rajendran, K. et al. (2020), examining the evidence from non-RCTs, concluded that convalescent plasma might be effective and safe for treating patients with COVID-19. However, it also called for RCTs to be done. |
|
Recent Guidelines |
1. IDSA: Among hospitalized patients with COVID-19, convalescent plasma is only recommended in the context of clinical trials.
2. NIH: Insufficient evidence to recommend for/against the use of convalescent plasma. 3. CMA recommends against the use of convalescent plasma in patients with COVID-19 (weak recommendation). 4. ANZICS: Not provided. |
Mesenchymal Stem Cells (MSCs, 27 studies, Table 3)
Table 3. An update on mesenchymal stem cells
| RCTs with results | 1. No RCT results available. |
| Recent Guidelines | 1. IDSA: Not provided.
2. NIH: Not provided. 3. CMA: Not provided. 4. ANZICS: Not provided. |
Tocilizumab (23 studies, Table 4)
| Table 4. An update on tocilizumab | |
|
RCTs with results |
1. No published peer-reviewed RCT results are available.
2. Results from an open-label RCT, involving 129 moderate to severe COVID-19 patients, were recently revealed (Link). It showed that a significantly lower proportion of patients in the tocilizumab arm required ventilation or deceased, compared to patients in the standard care arm. |
|
Recent Guidelines |
1. IDSA: Among hospitalized patients with COVID-19, tocilizumab is only recommended in the context of clinical trials.
2. NIH: Insufficient evidence to recommend for/against the use of tocilizumab. 3. CMA: Not provided. 4. ANZICS: Not provided. |
Lopinavir+Ritonavir (16 studies, Table 5)
| Table 5. An update on lopinavir+ritonavir | |
|
RCTs with results |
1. An open-label RCT published in the New England Journal of Medicine found no benefits with using lopinavir+ritonavir in patients with severe COVID-19, compared to standard care (Cao, B. et al. 2020).
2. A non-peer-reviewed RCT published in MedRxiv found no significant improvement in outcomes, such as time to positive-to-negative conversion of SARS-CoV-2 nucleic acid, between the lopinavir+ritonavir group and the no antiviral medication group (Li, Y. et al., 2020). 3. A multi-center, open-label RCT was recently published in the Lancet investigating the triple combination of lopinavir+ritonavir, interferon beta-1b, and ribavirin vs. lopinavir+ritonavir in hospitalized patients with COVID-19 (Hung, I. F. et al., 2020). The results showed that, compared to lopinavir+ritonavir alone, the triple combination therapy was superior in symptom relief and shortening the hospital stay in patients with mild to moderate COVID-19. |
|
Recent Guidelines |
1. IDSA: Among hospitalized patients with COVID-19, lopinavir+ritonavir are only recommended in the context of clinical trials.
2. NIH recommends against the use of lopinavir+ritonavir in treating COVID-19 except in the context of clinical trials. 3. CMA recommends against the use of lopinavir+ritonavir in patients with COVID-19 (weak recommendation). 4. ANZICS: Not provided. |
Favipiravir (11 studies, Table 6)
| Table 6. An update on favipiravir | |
|
RCTs with results |
1. No published peer-reviewed RCT results are available.
2. A recent non-peer-reviewed RCT in MedRxiv showed that favipiravir didn’t exhibit more benefits in outcomes (e.g., viral negative in day 14, time to clinical improvement) compared to standard care (Lou, Liu, & Qiu, 2020). |
|
Recent Guidelines |
1. IDSA: Not provided.
2. NIH: Not provided. 3. CMA recommends against the use of favipiravir in patients with COVID-19 (weak recommendation). 4. ANZICS: Not provided. |
Remdesivir (10 studies, Table 7)
| Table 7. An update on remdesivir | |
|
RCTs with results |
1. No published peer-reviewed RCT results are available.
2. A multinational, placebo-controlled RCT, known as the Adaptive COVID-19 Treatment Trial (ACTT), released its preliminary data recently, showing that hospitalized patients with COVID-19 who were randomized to remdesivir had a shorter time to clinical recovery than those who received placebo (Link). |
|
Recent Guidelines |
1. IDSA: Not provided.
2. NIH recommends the use of remdesivir for the treatment of hospitalized patients with severe COVID-19. For patients with mild to moderate COVID-19, remdesivir is recommended only in the context of clinical trials. 3. CMA: Not provided. 4. ANZICS: Not provided. |
References
Australia and New Zealand Intensive Care Society (ANZICS). (2020). COVID-19 Guidelines version 2. Retrieved from https://www.anzics.com.au/wp-content/uploads/2020/04/ANZI_3367_Guidelines_V2.pdf
Bhimraj, A., Morgan, R. L., Shumaker, A. H., Lavergne, V., Baden, L., Cheng, V., . . . Falck-Ytter, Y. (2020). Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Retrieved from https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., . . . Wang, C. (2020). A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. doi:10.1056/NEJMoa2001282
Chen, J., Liu, D., Liu, P., Xu, Q., Xia, L., Ling, Y., . . . Hongzhou, L. (2020). A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ [Chinese], 49(2), 215-219.
Chen, Z., Hu, J., Zhang, Z., Jiang, S., Han, S., Yan, D., . . . Zhang, Z. (2020). Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv, 2020.2003.2022.20040758. doi:10.1101/2020.03.22.20040758
Hung, I. F., Lung, K. C., Tso, E. Y., Liu, R., Chung, T. W., Chu, M. Y., . . . Yuen, K. Y. (2020). Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. doi:10.1016/S0140-6736(20)31042-4
Li, Y., Xie, Z., Lin, W., Cai, W., Wen, C., Guan, Y., . . . Li, L. (2020). An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). medRxiv, 2020.2003.2019.20038984. doi:10.1101/2020.03.19.20038984
Lou, Y., Liu, L., & Qiu, Y. (2020). Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: an Exploratory Randomized, Controlled Trial. medRxiv, 2020.2004.2029.20085761. doi:10.1101/2020.04.29.20085761
National Institute of Health. (2020). COVID-19 Treatment Guidelines. Retrieved from https://covid19treatmentguidelines.nih.gov/overview/
Rajendran, K., Krishnasamy, N., Rangarajan, J., Rathinam, J., Natarajan, M., & Ramachandran, A. (2020). Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. doi:10.1002/jmv.25961
Tang, W., Cao, Z., Han, M., Wang, Z., Chen, J., Sun, W., . . . Xie, Q. (2020). Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. medRxiv, 2020.2004.2010.20060558. doi:10.1101/2020.04.10.20060558
Ye, Z., Rochwerg, B., Wang, Y., Adhikari, N. K., Murthy, S., Lamontagne, F., . . . Guyatt, G. H. (2020). Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ. doi:10.1503/cmaj.200648
